In the Hall lab, we want to understand the role sensory experiences play in shaping early, postembryonic brain development. Postembryonic development is unique in that it encapsulates the period of time in which sensory systems first become sufficiently developed to start processing environmental input for the first time (for example, seeing, smelling, feeling, hearing, tasting). While sensory input is reaching the brain for the first time, the brain is still growing, through both changes in pre-existing brain cells and the generation of new brain cells (neurogenesis). This combination of sensory processing and continued brain growth make postembryonic development the period in life in which brain development is most affected by sensory experience. This postembryonic sensitivity to experience is thought to explain why certain types of learning, for example musical training on an instrument or learning a second language, are easier earlier than later in life.
Below, is a little bit about the model system we use in the lab as well as our current research aims. If you are a prospective student, however, know these approaches aren’t restrictive and that I am interested in any lines of research that seek to understand the role sensory experience plays in shaping early brain and behavioural development, particularly through the generation, recruitment, and survival of new brain cells.
Our model, the zebrafish
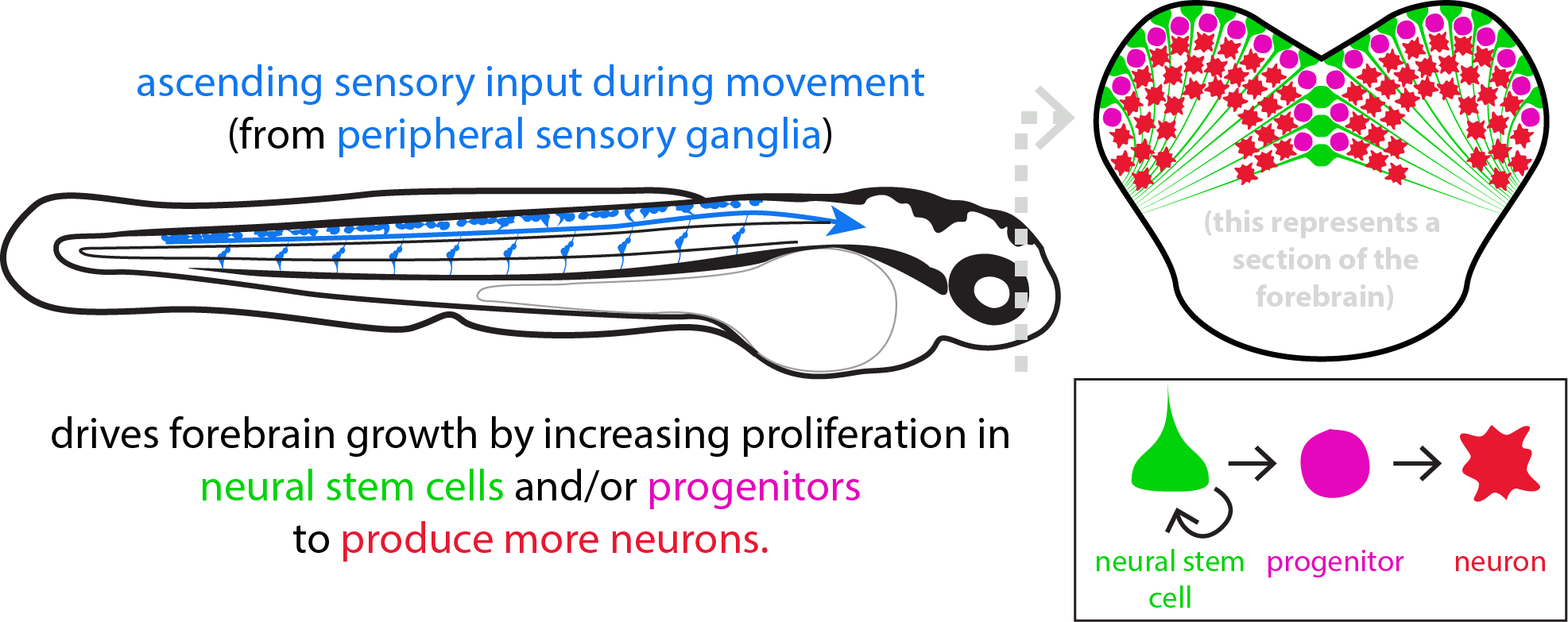
In my previous work, I found that sensory input associated with movement (swimming) drives increases in neurogenesis in the zebrafish forebrain. Through this form of movement-dependent neurogenesis, zebrafish larvae that swim the most exhibit the greatest amount of neurogenesis and grow the largest forebrains. I further found that this sensory input appears to be somatosensory in nature and is detected by dorsal root ganglia, bundles of sensory cells located along the body in vertebrates. Altogether, I believe this form of movement-dependent neurogenesis may represent a fundamental mechanism through which body/motor development can be coupled to brain growth and may explain why early forms of reduced movement (sedentariness early in life or muscular disease) are often accompanied by cognitive disabilities.
How does movement-related sensory input reach the forebrain?
Whereas our previous work has identified that dorsal root ganglia convey signals associated with movement up to the forebrain, these ganglia do not project the entire way there themselves. Instead, brain regions receiving dorsal root input act as intermediaries to relay the signal to the forebrain, ultimately affecting brain growth. Our first research aim is to identify the circuits within the brain through which movement regulates forebrain neurogenesis.
An ongoing project serving this research aim is to isolate dorsal root ganglia transgenically, which can be imaged in their entirety in the live zebrafish larvae! By characterizing the projections of dorsal roots into the brain, we can identify the next step in the neural circuit regulating movement-dependent neurogenesis. The ultimate goal of this project is to identify a whole neural circuit capable of modulating neurogenesis in response to changes in sensory input.
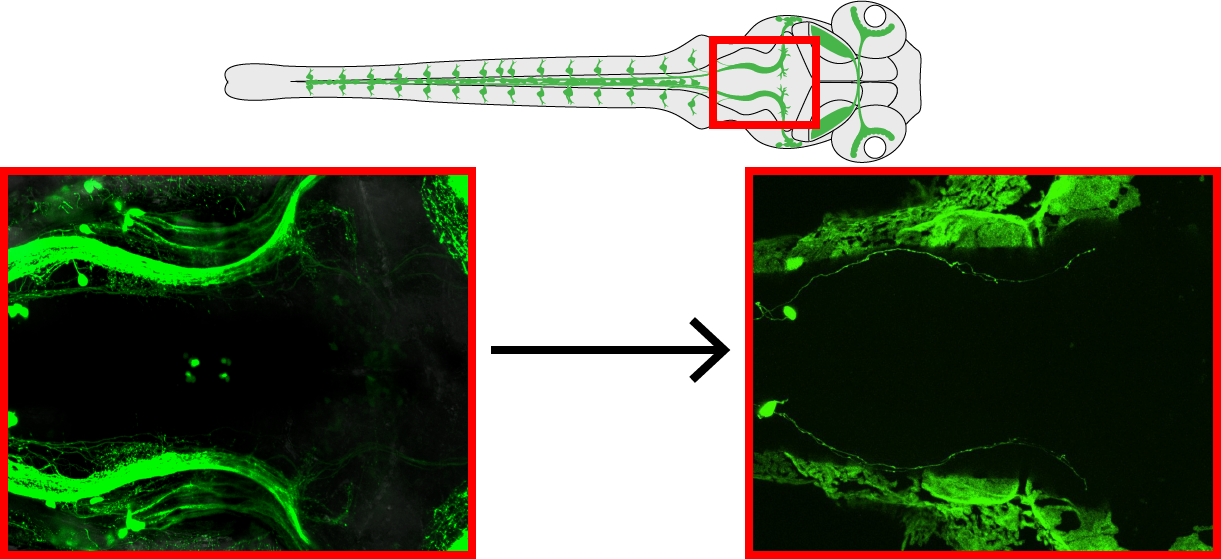
By using transplantation techniques, we can “dilute” the transgenic signal of green fluorescent protein from a full expression pattern (left) to specific expression in isolated dorsal root ganglia (right).
How does sensory experience modulate the process of neurogenesis?
Once ascending sensory input reaches the forebrain, how does it influence neurogenesis? This question is more complicated than it may seem because neurogenesis encapsulates a process that includes the proliferation, differentiation, and survival of new neurons. Our second research aim is to uncover how sensory experience regulates these different neurogenic processes.
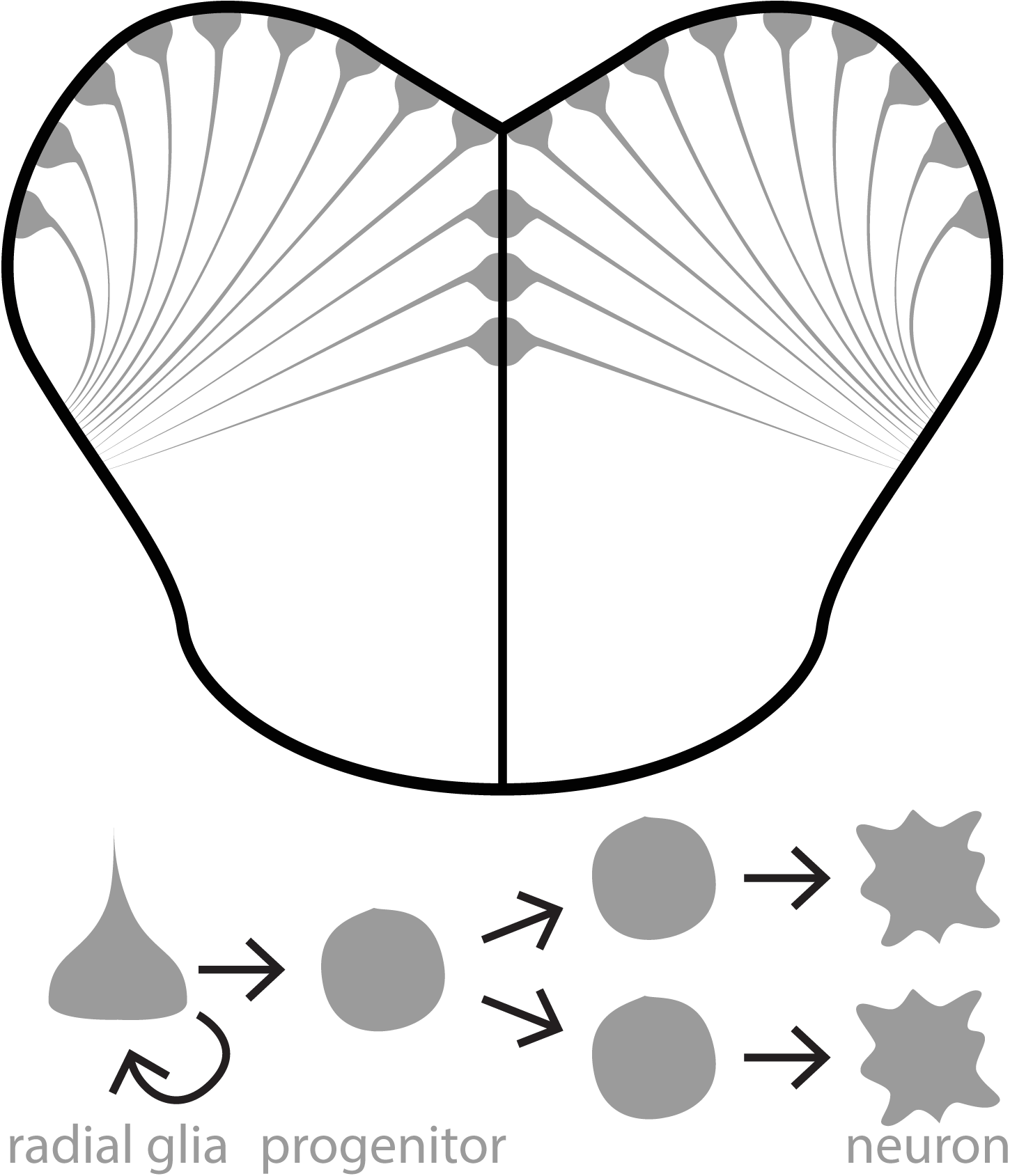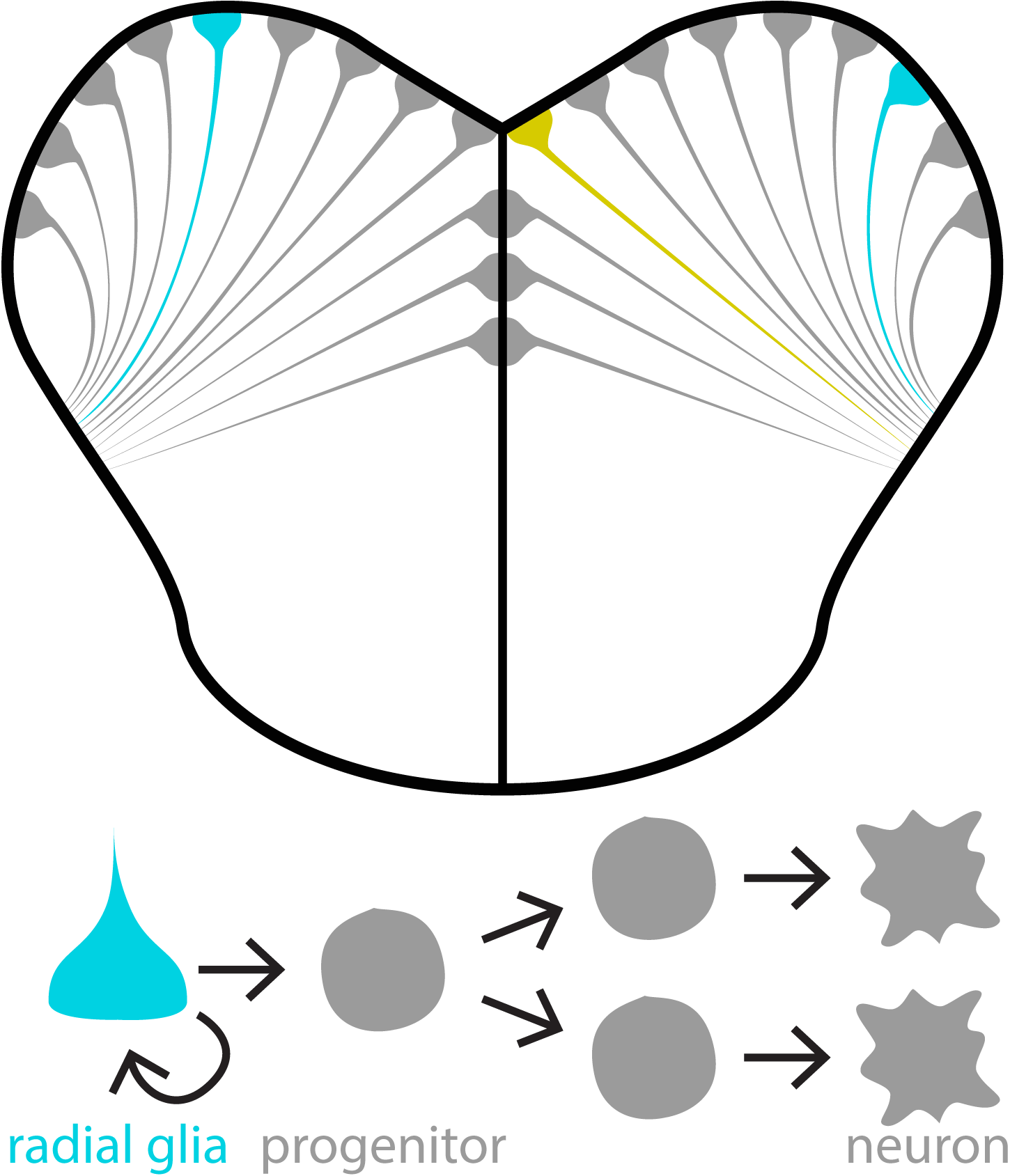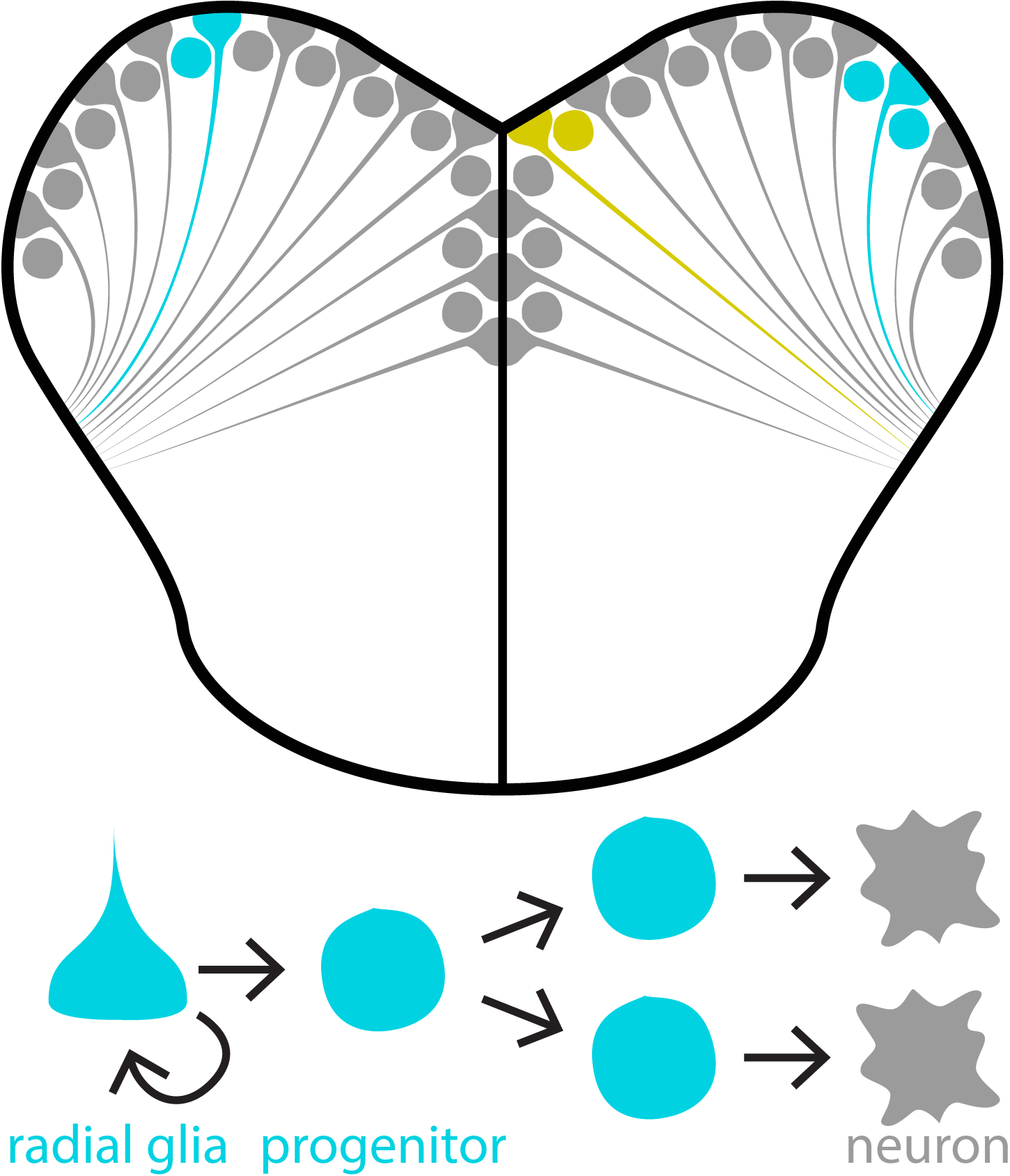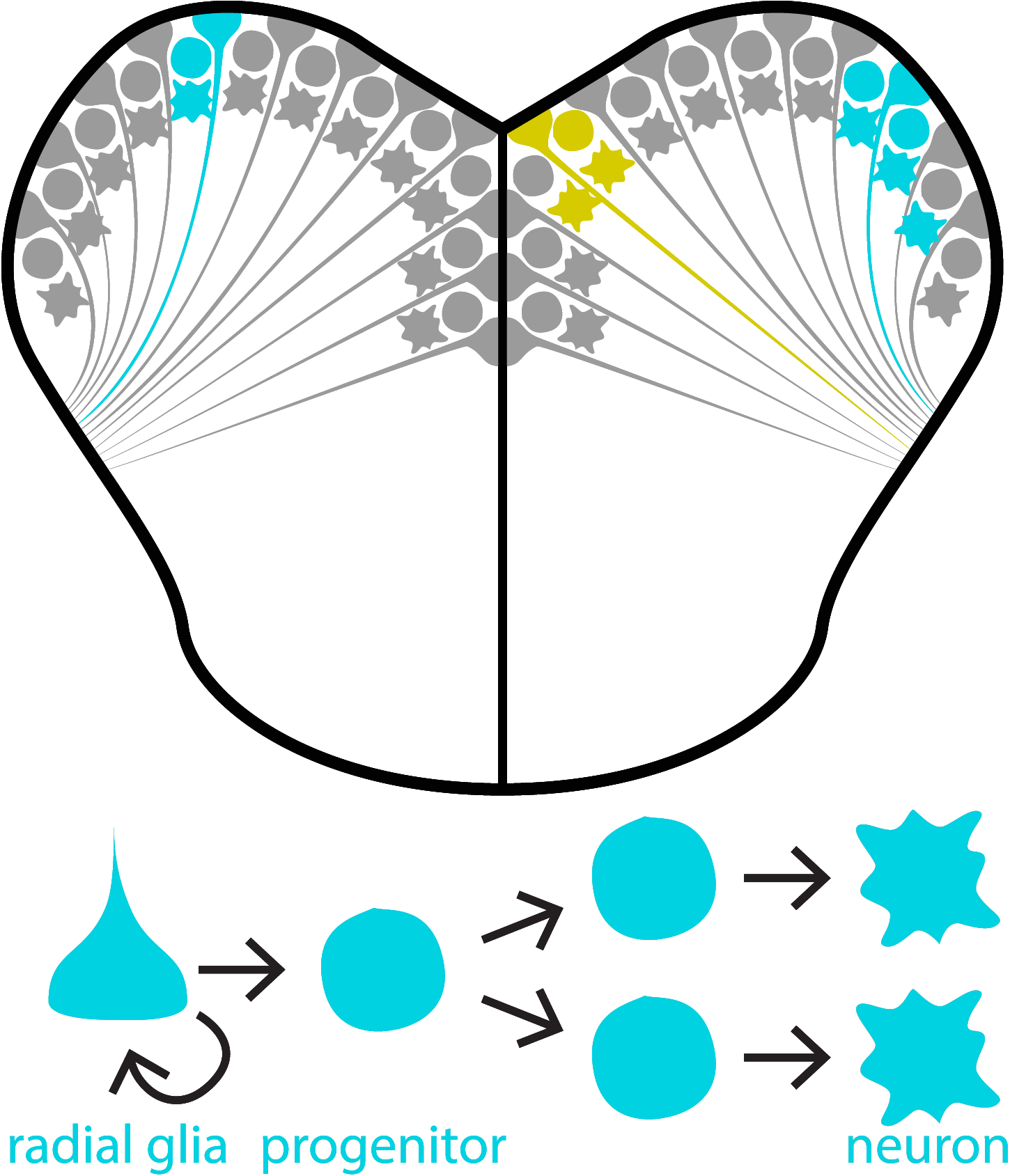
One exciting approach within this research aim is to use cell lineage tracing techniques. Using transgenic zebrafish, we can induce a fluorescent colour change in neural stem cells (depicted here as cyan or yellow fluorescence). This colour change is inherited by progenitors and neurons derived from “induced” neural stem cells. Using this approach we can examine how sensory experiences modulate the types and numbers of cells produced in each neural stem cell lineage. We also produce pretty nice looking micrographs, as well!
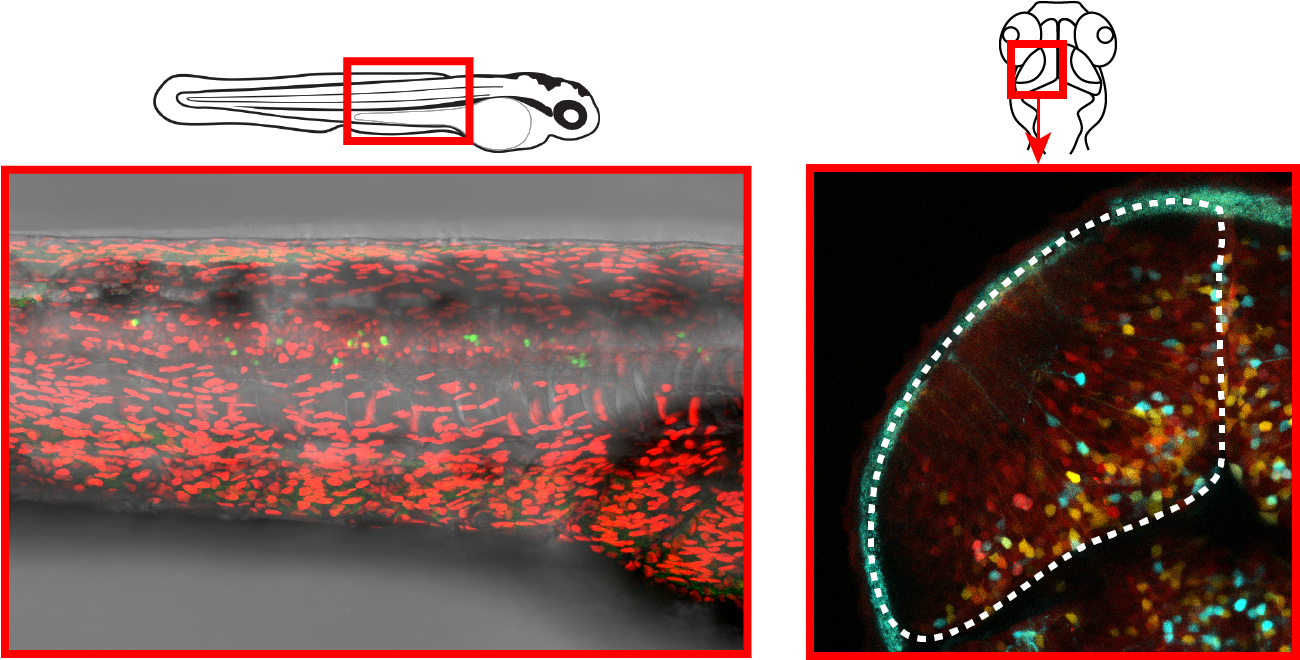
Examples of neural stem cell coloured lineages in the spine (left; green cells) and midbrain (right; cyan and yellow cells).
What role do new neurons serve in the postembryonic brain?
Although our lab is contributing to a growing body of work demonstrating that sensory experience has the capacity to shape brain development by regulating neurogenesis, we know little about the roles these new cells serve early in life. This project focuses on both developing tools to modulate the process of neurogenesis in the zebrafish larvae and creating new behavioural paradigms through which the consequences of impaired/enhanced neurogenesis can be tested in our larvae.
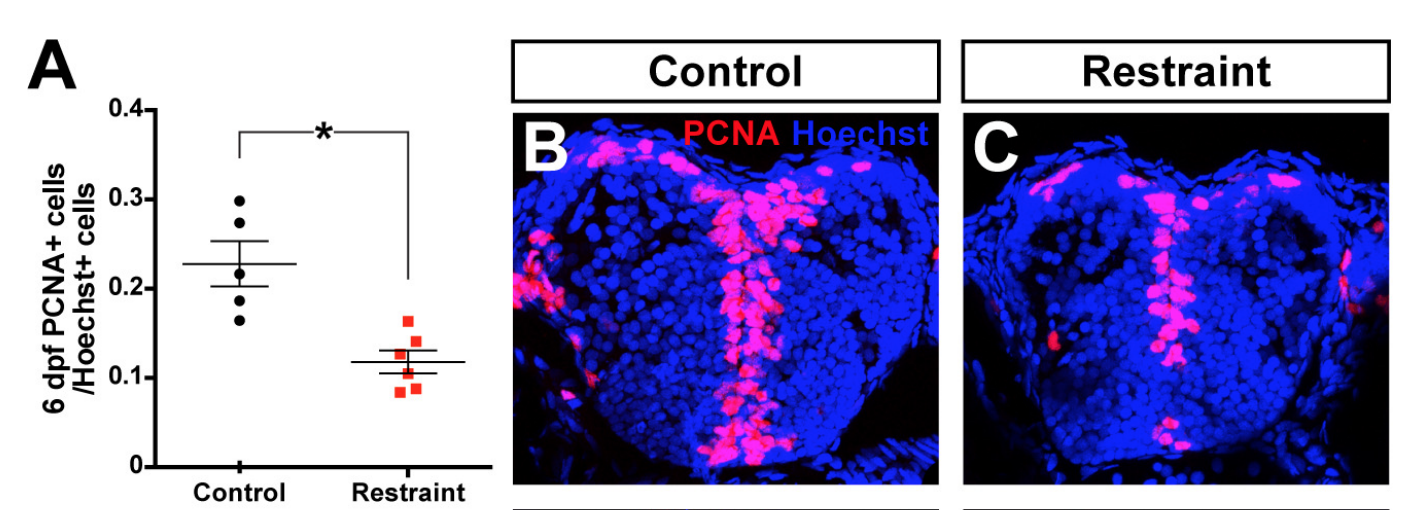
Whereas we have found that reducing larval swimming by restraint significantly reduces the amount of neurogenesis (PCNA+ proliferative cells parked in pink), the consequences of developing a brain with fewer neurons is largely unknown. (Figure from Hall and Tropepe, 2018; eLife)